Abstract
Background: Advanced light-chain (AL) cardiac amyloidosis portends dismal prognosis. Daratumumab has shown rapid, deep and high rate of response in AL amyloidosis with favorable safety. But late-staged patients were excluded from most trials. Clinical data about daratumumab in high-risk patients is still lacking.
Aims: To prospectively evaluate the efficacy and safety of daratumumab plus bortezomib and dexamethasone in high-risk patients with Mayo 2004 stage 3 AL amyloidosis.
Methods: This is a prospective phase 2, open-label and single-arm clinical trial in newly diagnosed patients with Mayo 2004 stage 3a and 3b AL amyloidosis at Peking Union Medical College Hospital (Beijing, China). All 40 patients should have baseline dFLC >50mg/L and receive 12 cycles of treatment. The treatment scheme includes daratumumab at 16 mg/kg iv weekly during cycles 1-2, once every two weeks during cycles 3-6 and once every 4 weeks during cycles 7-12, bortezomib at 1.3mg/m2 subcutaneously weekly during cycles 1-6 and dexamethasone at 20mg weekly iv/po during cycles 1-6. The primary outcome is hematological response rate of ≥VGPR at 3 months. (NCT04474938)
Results: From May, 2021 to March, 2022, 40 patients were enrolled. Thirty (75%) patients were male and the median age was 59 years (range 40-77). Twenty patients (50%) were stage 3b. The median NT-proBNP was 7801 ng/L (range 803 ~ 35000) and the median cTnI was 0.17μg/L (range 0.07-3.07, one with cTnI <0.1μg/L had baseline cTnT of 0.035μg/L). Median dFLC was 274 mg/L (range 72-2966). Twenty-four patients (60%) had NYHA class III or IV heart function. Twelve (30%) patients had renal involvement and ten (25%) patients had liver involvement.
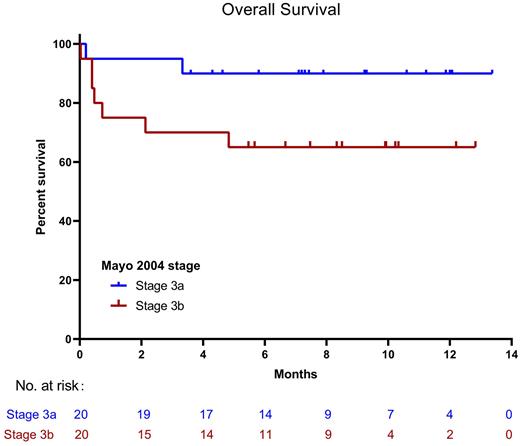
Up to July 3rd, 2022, the median number of treatment cycles was 8 (range 0.25-12). The hematological response rate of ≥VGPR at 3m was 67.5%, including 45% CR and 22.5% VGPR, and ORR was 82.5%. 52.5% of patients reached stringent dFLC response. Of 9 patients who have completed 12 cycles of treatment, 6 cases (66.7%) had negative MRD by multi-parametric flow cytometry (sensitivity 1×10-5). Cardiac response rate was 37.5% (1 CR, 2 VGPR and 12 PR) at 3m and 38.2% (1 CR, 7 VGPR and 5 PR) at 6m. After the median follow-up time of 9 months (range 4-13), 9 patients died because of disease progression. Early mortality is 15% within 1m and 17.5% within 3m. Three patients withdrew due to cardiac progression and 1 patient withdrew due to hematological progression. The 6-month survival rate of all patients was 77%, with 90% for stage 3a and 65% for stage 3b. Median OS was not reached.
Common grade 3 adverse events included infection (15%), diarrhea (7.5%), emesis (5%), intestinal obstruction (5%), weakness (2.5%), peripheral neuropathy (2.5%), DVT (2.5%), GI bleeding (2.5%), anemia (2.5%), pneumothorax (2.5%), ischemic stroke (2.5%) and infusion reaction (2.5%). Grade 4 diarrhea was recorded in 1 patient and improved after symptomatic treatment. Four cases, 1 case and 1 case suspended bortezomib due to diarrhea, intestinal obstruction, and acute cholecystitis, separately. One patient received reduced-dose bortezomib because of diarrhea. No patients withdrew due to adverse events.
Summary/Conclusion
Daratumumab plus bortezomib and dexamethasone had favorable safety and efficacy among patients with stage 3 cardiac AL amyloidosis, but high early mortality is still an important obstacle to management of high-risk patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal